3 November 2012
Introduction
On this day, I observed many of my cultures macroscopically and microscopically, and was able to identify two genera.
Observations
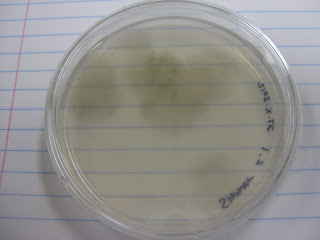
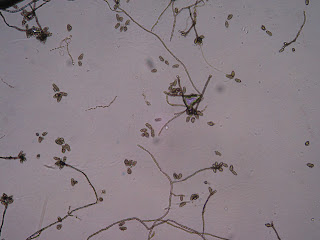
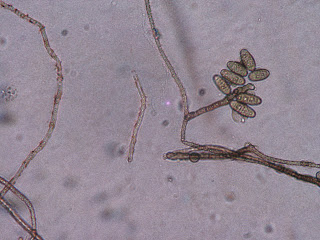
I first examined my wasp nest plate (Fig. 1), which contained many different colonies (Fig. 2). However, when observing these colonies microscopically, most of them appeared to be of the Aspergillus genus (Figs. 3, 4).
 |
Fig. 1
Wasp nest plate |
 |
Fig. 2
A variety of different colonies on the wasp nest plate |
 |
Fig. 3
Taken from wasp nest colony, appears to be Aspergillus |
 |
Fig. 4
Another sample taken from wasp nest colony, appears to be Aspergillus |
One seafoam green colony, however, did appear to be different from the others microscopically (Figs. 5-9).
 |
Fig. 5
Conidia from seafoam green colony on wasp nest plate |
 |
Fig. 6
Conidiophore from seafoam green colony on wasp nest plate |
 |
Fig. 7
Conidiophore from seafoam green colony on wasp nest plate |
 |
Fig. 8
Conidia from seafoam green colony on wasp nest plate |
 |
Fig. 9
Yet another strange-looking conidia from seafoam green colony on wasp nest plate |
I next observed the cricket isolation plate (Fig. 10). I'm not sure about the conidiophores in Figs. 11-13, but I'm pretty sure that Figs. 14-16 show a common Zygomycete and perhaps
Aspergillus.
 |
Fig. 10
Cricket isolation plate |
 |
Fig. 11
Conidiophores from cricket isolation |
 |
Fig. 12
Conidiophores from cricket isolation |
 |
Fig. 13
Conidiophores from cricket isolation |
 |
Fig. 14
Putative Zygomycete |
 |
Fig. 15
Aspergillus sp? |
 |
Fig. 16
Aspergillus sp? |
I next observed the shower mold again. This time, I was only looking at the plate which contained the agar block transferred from the original plate. When observing this under the microscope, it was clear that chlamydospores were present (Figs. 17-19). However, I was not able to identify the genus at this time.
 |
Fig. 17
Chlamydospores from shower plate |
 |
Fig. 18
Chlamydospores from shower plate |
 |
Fig. 19
Chlamydospores from shower plate |
Aaaannnnddd finally.....
At the very end of the day, I was finally able to catch a break by identifying two very easily recognizable genera (thank gods!).
First, I took the plate containing the large black fungus I scraped from a tree in Longview. I had not looked at this plate since I made it, so I was curious to see what I would find. It turns out that both
Curvularia (Fig. 20) and
Pestalotia sp. (Fig. 21) were present on the plate on separate sides. I apologize for the extremely poor quality of the photographs; my camera died right before I identified these, so I had to use my iPhone (which is very hard to use for this purpose).
 |
Fig. 20
Curvularia sp. |
 |
Fig. 21
Pestalotia sp. |
The very last thing I did on this day was plate fungus from a piece of decaying wood that had green mycelia growing inside (Fig. 22). It appeared as though ants were farming this fungus. I also plated fungus from a watermelon leaf (Fig. 23). Both were plated onto 1/2 strength PDA.
 |
Fig. 22
Decaying wood with green mycelia inside |
 |
Fig. 23
Watermelon leaf with white fungus on the surface |
Conclusion
I am very happy to have 2/3 unknowns identified. Hopefully I can get the last one soon!
All for now,
C